The elasticity diagram provided by Material Property IOANA, gives an excellent explanation of what happens to food that provides its texture. This is called elasticity. This term is a continuation of my previous article, which was about mouthfeel. Elasticity affects the texture and mouthfeel of a certain food item. In the diagram above, the strands of gluten increase the elasticity of any food item. The more strands there are, the higher the elasticity, or, as a food scientist would call it, the elastic modulus. For example, when you cook a steak, the protein bond length in the steak shortens because water is expelled during cooking. This, in turn, leads to an increase in bond density and elastic modulus. This is usually true for foods that have different textures, as well as, for foods before and after cooking. When the food is deformed in cooking, it changes the position of the molecules in that food. Making bonds between molecules larger or smaller changes in the distance between the bonds. When you compare steak to gluten above. There are a greater number of bonds (bond density) in the steak than in gluten. Hence, gluten has a smaller elasticity (elastic modulus) than steak for a given volume. You have to compress fewer bonds when you press on bread, so less energy is required to deform it. In steak, the protein gets denatured and then coagulates, causing an increase in bonds between unfolded proteins. This, in turn, leads to greater bond density and elastic modulus. Water loss and protein contraction also play a role in increasing the density of bonds.
Continue readingMonthly Archives: March 2018
Gelatinization of starches

Image credit: Cartoonstock by Baloo
The diagram above is a fun way to start the topic today. Gelatinization is such an interesting and important topic in baking and cooking. Starch is used in most food items which have different uses.
Let us start by defining it. What is starch? it is a complex carbohydrate, a polysaccharide. It forms a single glucose molecule linked together in a branched and a linear fashion. Feel free to read up on the structure if you are interested to learn more. Also, under references, you can read up on starches.
Now that you know what a starch molecule is. Let us talk more about what happens to the starch when it is cooked. The diagram below gives a simple overview of what happens, which I like, especially if you are a visual learner.
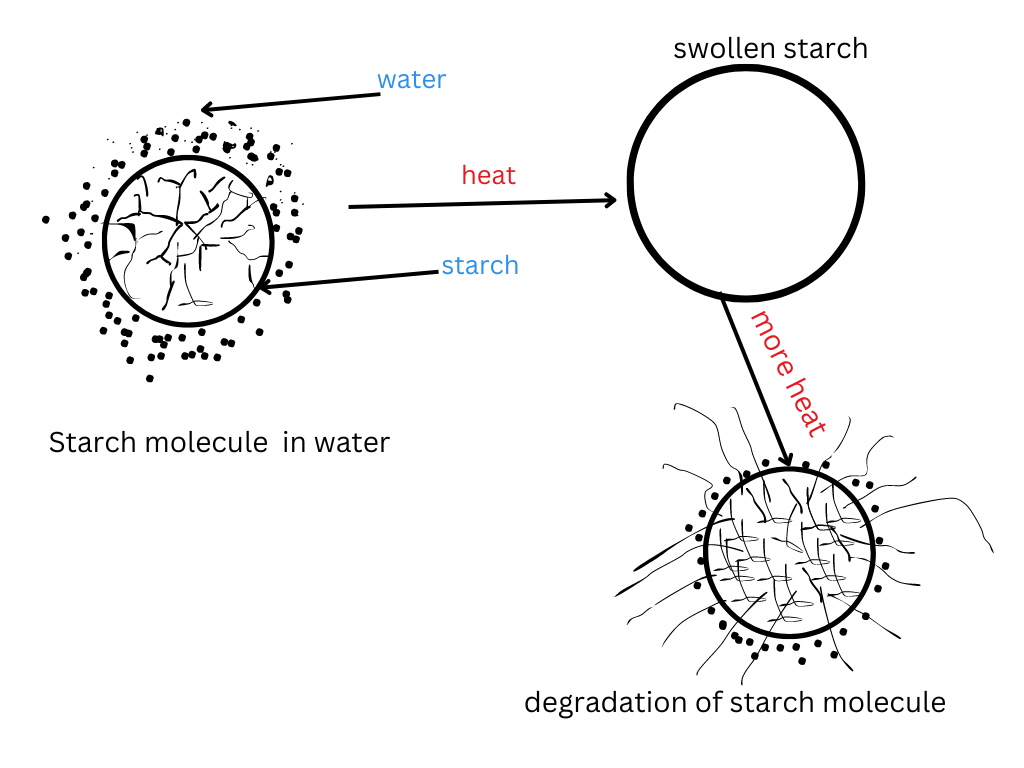
The diagram above gives a summary of what happens during gelatinization. First, the starch is subjected to heat in proximity of water. As a result, the water slowly gets absorbed by the starch molecule, due to lose of structure. In turn, gradual swelling occurs, with thickening of liquid as water gets trapped. Lastly, the granule bursts due to an increase in pressure. This is due to a lower in pressure in the solution compared to the starch molecule. Other factors that cause degradation of the starch molecule include, increase in heat which leads to loss of thickening. The whole process is called starch gelatinization. This happens with every product that has starch but in different ways. For example, when you boil pasta in water, the starch makes the product soft. Another example, starch in instant pudding has a similar effect of making the pudding thicker. Therefore, you can find starch in each product, but the uses differ for each one.
Continue readingMouthfeel, smell and taste
Image credit: Cooking with Sin
Creative looking picture, don’t you think? is your mouth watering yet? well, if it is you’re ready for food! You scoop the cream, ice cream, or whatever it is. How does the sample feel in your mouth? the word that food scientist refers to as mouthfeel. As the picture below continues, there are so many synonyms for mouthfeel.
Continue readingThickening agents

Image credit: Pexels by SD CREATIVES PH
Ever wondered what causes the food you eat is thick? or soft? an ingredient called a thickening agent contributes to this, which affects the flow behavior (viscosity), mechanical solid property (texture), and mouthfeel. A thickening agent can either be polysaccharides (starches, vegetable gums, and pectin), or proteins. We find it in food from ice cream, flour, sauces, soups, gravies, salad dressings, and toppings.
Continue readingThe Maillard Reaction

Image by Public Domain Pictures
“For British food scientists, toast color is no longer a matter of personal preference—it’s a matter of health.”
The smell of fresh bread, or cooked crispy meat. Makes you wonder how does that happen? Each food product you cook with in the kitchen has two ingredients, proteins (amino acid) and sugars (reducing sugars) that lead to a reaction, which creates flavors and aroma. The most important in the reaction is heat. To start the reaction, the temperature needs to be above 300F to evaporate the protein on the surface of the product. This is an important first step because there are no enzymes that assist in the reaction. This process is called the Maillard reaction.
Continue reading